How can I tell what type of plastic something is made of, and if that plastic is safe?
There is a way to identify the type of plastic in many everyday products, especially food storage containers and packaging. Many, but not all, such plastic products have a number – the "resin identification code" – surrounded by a chasing arrows symbol and molded, formed or imprinted in or on the container, often on the bottom. Use of the word "resin" is synonymous with "polymer" or "plastic type."
A few things to keep in mind about these resin identification codes:
- Developed by the plastics industry to faciliate recycling. This system of coding was developed in 1988 by the U.S.-based Society of the Plastics Industry to facilitate the recycling of post-consumer plastics. It is voluntary for plastic manufacturers, but has become relatively standard on certain plastic products sold globally.
- Codes do not guarantee recyclability. Although this coding system is designed to facilitate recycling, the presence of a code on a product does NOT mean it is recyclable. We provide below information on the estimated recycling rate of each plastic resin.
- Codes do not indicate toxicity or safety. The codes do not provide any information on the toxic chamicals contained in the identified plastics or whether or not they are safe - the code simply identifies the type of plastic resin. Plastic manufacturers are not required to disclose other chemicals that have been added to the plastic polmer. Most plastics have numerous synthetic, often petroleum-derived additives in them. We provide below information on what we perceive to be the toxicity and safety of each plastic type, based on our research, experience, and practice of the precautionary principle.
- Only six plastic types are explicitly identified. Codes #1 to #6 each identify a specific plastic polymer commonly used in consumer goods all over the world. Code #7 is a general catch-all category which is essentially for every other type of plastic. We highlight a couple of the key common plastics that fall into this category.
The seven plastic resin identification codes are laid out below with added information describing characteristics of each plastic type, typical products it is found in, our perception of its toxicity and safety (including whether or not to avoid it), its estimated recycling rate and recycled products made from it, and suggestions for alternatives you can use to replace it in everyday life (including things available in our store).
We hope that being aware of this system and these plastic types will help you better assess the plastics in your everyday life and the potential risks associated with their use.
Here's a quick summary of our suggestions for use of these plastics, particularly if using them for food and drink (see below for details):
- 2, 4 and 5 are OK for limited use
- AVOID 1, 3, 6, and 7 (polycarbonate)
 Polyethylene terephthalate (PET or PETE or polyester)
Polyethylene terephthalate (PET or PETE or polyester)
Description: PET is the most well known member of the polyester family of plastic polymers. It initially gained widespread use as a wrinkle-free fiber (commonly called "polyester"), and the majority of its production still goes toward textile manufacturing. It has become extremely popular for food and drink packaging purposes because of its strong ability to create a liquid and gas barrier - so oxygen cannot get in to spoil food, and the carbon dioxide that makes drinks fizzy cannot get out. Properties: clarity, lightness, strength, toughness, barrier to liquid and gas.
Typical Use: Bottles (water, soft drink, juice, beer, wine, mouthwash, salad dressing), peanut butter/jam jars, oven-ready and microwaveable meal trays, detergent and cleaner containers. Also used in liquid crystal displays, film for capacitors, insulation for wire and insulating tapes, and as a common finish for wood products such as guitars, pianos and vehicle/yacht interiors.
PET fabric (polyester) is commonly used in textiles (fabric and clothing), padding and insulation (for pillows, comforters, upholstery), carpet, and mouldings. Also for tyre reinforcements, conveyor belts, safety belts, coated fabrics and tarpaulins.
Toxicity: PET may leach antimony (antimony trioxide is used as a catalyst and flame retardant in PET) (PET1, PET2). The longer a liquid is left in a PET container the greater the potential for release. As well, warm temperatures inside cars, garages, and enclosed storage areas increase the release of antimony into the liquid. Antimony trioxide is considered a possible carcinogen (PET3). Workers exposed to antimony trioxide for long periods of time have exhibited respiratory and skin irritation and among female workers, increased incidence of menstrual problems and miscarriage -- while there is no evidence that these effects could arise from exposure to the small amounts of antimony released from PET products (such as water bottles), we prefer not to be exposed to it at all (PET3).
Evidence is also emerging that phthalate endocrine disruptors also leach from PET (PET4, PET5).
PET as a textile - i.e., polyester - likely contains flame retardants incorporated into it during the manufacturing process. As such, polyester is often described as "inherently flame retardant", but it is unclear exactly which flame retardant chemicals are added to polyester as it is being made, and thus it is difficult to know if there is a toxicity issue with polyester fibre.
Recycling: About 29% (PET6). Recycled material downcycled into polyester fibre for fleece clothing, tote bags, strapping. Note: "Downcycling" means that the recycled material is of lower quality than the original PET, and can only be made into progressively lower quality products until it can no longer be recycled and becomes landfill waste which is most likely landfilled.
Alternatives: Use a glass or stainless steel reusable water bottle. Buy in glass and reuse those bottles/jars - mason jars are incredibly versatile. Choose natural fabrics (e.g., organic cotton, wool, hemp) for clothing.
Our Suggestion: AVOID. Many consider PET a relatively safe single use plastic, but given the research indicating it can release antimony and phthalates, and our precautionary approach, we suggest avoiding it whenever possible. If you must use it, keep it away from heat and do not reuse it.
 High density polyethylene (HDPE)
High density polyethylene (HDPE)
Description: Polyethylenes are the most widely used family of plastics in the world. The versatile polyethylene polymer has the simplest basic chemical structure of any plastic polymer (repeating units of CH2: one carbon and two hydrogen molecules) making it very easy to process and thus extremely popular for numerous low value applications - especially packaging. HDPE has long virtually unbranched polymer chains which align and pack easily making it dense and very crystalline (structurally ordered) and thus a stronger, thicker form of of polyethylene. Properties: stiffness, strength, toughness, resistance to moisture, permeability to gas, ease of processing.
Typical Use: Plastic bags (grocery), opaque milk, water, and juice containers, bleach, detergent and shampoo bottles, garbage bags, dishes, yogurt and margarine tubs, cereal box liners, some medecine bottles. Also used in Tyvek insulation, PEX piping, plastic/wood composites.
Toxicity: Being relatively stable, it is generally considered a safer plastic for food and drink use, although some studies have shown that it can leach the endocrine disruptor nonylphenol (added to HDPE as a stabilizer), especially when exposed to ultraviolet light - i.e., sunlight - and possibly other additive chemicals with estrogen-mimicking activity (HDPE1, HDPE2, HDPE3).
Recycling: About 29% (HDPE4). Recycled material made into bottles for non-food items like shampoo, laundry detergent, motor oil; plastic lumber and furniture, piping, recycling bins, fencing, floor tiles, buckets, crates, flower pots, garden edging, film and sheeting.
Alternatives: Use glass or stainless steel reusable bottles and food storage containers. Buy in glass and reuse those bottles/jars - mason jars are incredibly versatile. Use reusable bags made of natural fibres (cotton, hemp).
Our Suggestion: RELATIVELY SAFE. But has been shown to release endocrine disrupting chemicals.
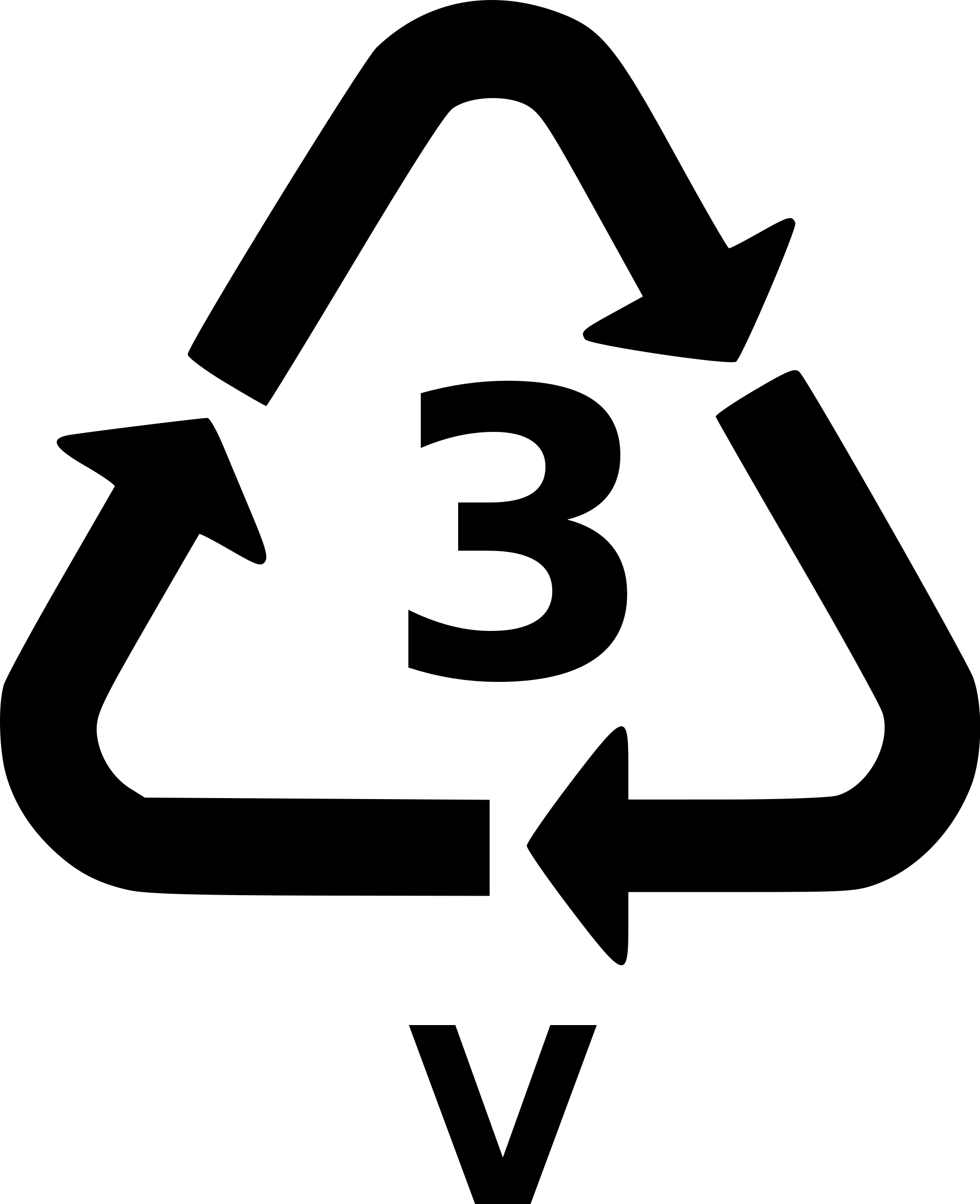 Polyvinyl chloride (V or Vinyl or PVC)
Polyvinyl chloride (V or Vinyl or PVC)
Description: Long the second most widely used plastic resin in the world (after polyethylene), PVC (or vinyl) use has decreased because of serious health and environmental pollution issues associated with its manufacture, use and disposal -- it's whole life cycle is toxic. But it is still popular and in common use because of its cost-effective versatility. The base monomer is vinyl chloride - the presence of chlorine is the cause of many of PVC's problems - which can be combined and blended with numerous chemicals (including plasticizers such as phthalates) to create resins with properties ranging from rigid to filmy to soft to leathery. Properties: versatility, ease of blending, strength, toughness, clarity, transparency.
Typical Use: Soft PVC (softened with plasticizers) used in toys, clear food (e.g., take-out) and non-food packaging (e.g., blister wrap, cling wrap), squeeze bottles, shampoo bottles, mouthwash bottles, cooking oil and peanut butter jars, detergent and window cleaner bottles, loose-leaf binders, shower curtains, blood bags and medical tubing, "pleather" clothing, Naugahyde upholstery, wire and cable insulation, carpet backing and flooring. Rigid PVC used for blister packs and clamshell packaging, credit cards, piping (e.g., for plumbing), vinyl siding, window frames, fencing, decking, and other construction materials.
Toxicity: PVC is widely considered the most toxic and hazardous plastic that is still - unbelievably so - commonly used to make numerous consumer products (PVC1, PVC2). It may contain and/or leach a variety of toxic chemicals including, but not limited to: bisphenol A (BPA), phthalates, lead, dioxins, mercury, and cadmium. Here is a taste of the toxic life cycle of PVC:
- The vinyl chloride monomer from which PVC is made is a known carcinogen (PVC3), thus putting manufacturing workers and surrounding communities at risk (PVC1).
- Soft forms of PVC, such as toys and packaging and bottles, may leach phthalates. For example, di(2-ethylhexyl) phthalate (DEHP) and butyl benzyl phthalate (BBzP) are two phthalates commonly used as plasticizers or softening agents (usually DEHP). DEHP and BBzP are endocrine disruptors mimicking the female hormone estrogen and have been strongly linked to asthma and allergic symptoms in children living in homes where PVC dust was present (PVC4, PVC5) and to ADHD in children (PVC6); may cause certain types of cancer, including breast cancer (PVC7). Recent consumer product legislation in Europe, Canada and the US, bans DEHP and BBzP and other dangerous phthalates from use in children's products in concentrations greater than 0.1% (PVC8, PVC9, PVC10).
- When PVC is burned (e.g., via waste incineration, car or home fires), dioxins are formed. Dioxins are known human carcinogens and persistent organic pollutants, and are considered one of the most toxic types of chemicals ever tested (PVC1, PVC11).
Recycling Rate: Very low (PVC12). Rarely recycled because it is difficult to do so on an industrial scale. It should not be recycled because it contaminates the recycling stream. Recycled PVC can become packaging, binders, decking, paneling, insulation, mud flaps, film and sheet, flooring, garden hoses.
Alternatives: Use glass or stainless steel reusable bottles and food storage containers. Buy in glass and reuse those bottles/jars - mason jars are incredibly versatile. Use non-plastic food wrap. Use recycled cardboard binders. Use recycled kraft paper, recycled cellulose wadding or compostable cornstarch peanuts for packaging. Use hemp or cotton shower curtains, and rubber hoses. Green building has taken off and there are now numerous healthy and eco-friendly alternatives to vinyl construction materials.
Our Suggestion: AVOID. If at all possible. Can be extremely toxic.
 Low density polyethylene (LDPE)
Low density polyethylene (LDPE)
Description: Polyethylenes are the most widely used family of plastics in the world. The versatile polyethylene polymer has the simplest basic chemical structure of any plastic polymer (repeating units of CH2: one carbon and two hydrogen molecules) making it very easy to process and thus extremely popular for numerous low value applications - especially packaging. LDPE polymers have significant chain branching including long side chains making it less dense and less crystalline (structurally ordered) and thus a generally thinner more flexible form of of polyethylene. Properties: strength, toughness, flexibility, resistance to moisture, ease of sealing, ease of processing.
Typical Use: Mostly for film applications like bags (grocery, dry cleaning, bread, frozen food bags, newspapers, garbage), plastic wraps; coatings for paper milk cartons and hot & cold beverage cups; some squeezable bottles (honey, mustard), food storage containers, container lids. Also used for wire and cable covering.
Toxicity: Being relatively stable, it is generally considered a safer plastic for food and drink use, although some studies have shown that it can leach the endocrine disruptor nonylphenol (added to LDPE as a stabilizer), especially when exposed to ultraviolet light - i.e., sunlight - and possibly other additive chemicals with estrogen-mimicking activity (LDPE1, LDPE2).
Recycling Rate: Low (LDPE3). Difficult to recycle. Recycled material can be made into compost bins, paneling, plastic lumber.
Alternatives: Use glass or stainless steel reusable bottles and food storage containers. Buy in glass and reuse those bottles/jars - mason jars are incredibly versatile. Use reusable bags made of natural fibres (cotton, hemp). Use non-plastic food wrap.
Our Suggestion: RELATIVELY SAFE. But has been shown to release endocrine disrupting chemicals.
 Polypropylene (PP)
Polypropylene (PP)
Description: Polypropylene is used for similar applications as polyethylenes, but is generally stiffer and more heat resistant - so is often used for containers filled with hot food. It too has a simple chemical structure (many methyl groups of CH3 - one carbon and three hydrogen molecules) making it very versatile. It's crystallinity (structural order affecting hardness & density) is quite high, somewhere between LDPE and HDPE. Properties: strength, toughness, resistance to heat, chemicals, grease & oil, barrier to moisture.
Typical Use: Food containers (ketchup, yogurt, cottage cheese, margarine, syrup, take-out), medicine containers, straws, bottle caps, Britta filters, Rubbermaid and other opaque plastic containers, including baby bottles. Other uses include disposable diaper and sanitary pad liners, thermal vests, appliance parts and numerous car parts (bumpers, carpets, fixtures).
Toxicity: Being relatively stable, it is generally considered a safer plastic for food and drink use, although it has been shown to leach plastic additives (such as the stabilizing agent oleamide) when PP labware was used in scientific experiments (PP1) and one older study has suggested heated PP may be linked to occupational asthma based on the exposure of a worker in a PP factory (PP2).
Recycling Rate: Low, because often pigmented or mixed with other resins, therefore difficult to sort (PP3). Recycled material made into brooms, brushes, bins pallets, auto battery cases, flower pots.
Alternatives: Buy in glass and reuse those bottles/jars - mason jars are incredibly versatile. Use a glass or stainless steel reusable water bottle. Purchase margarine/butter in cubes.
Our Suggestion: RELATIVELY SAFE. But has been shown to release additive chemicals when used as labware in scientific experiments.
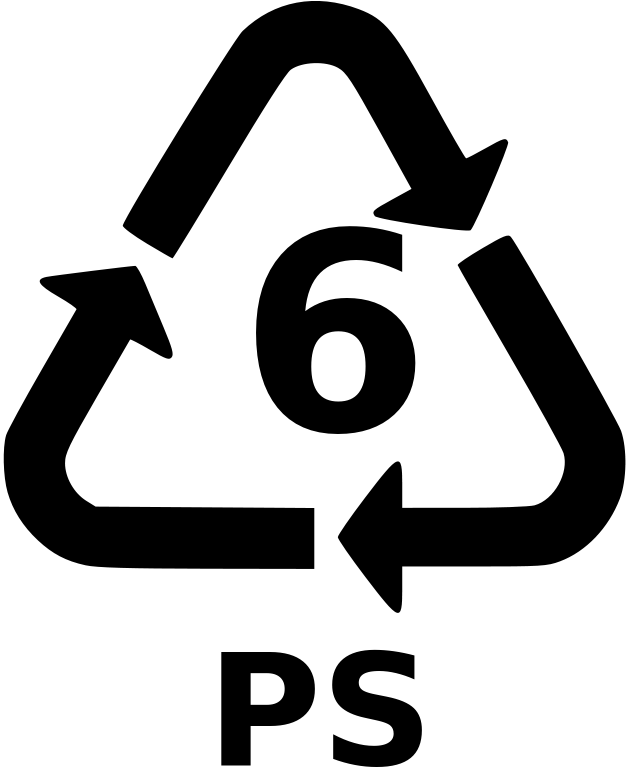 Polystyrene (PS)
Polystyrene (PS)
Description: Polystyrene is commonly associated with the trade name Styrofoam food containers and packing peanuts made of expanded PS (EPS), which is essentially foamed PS that has been puffed up with air. PS synthesis requires benzene, a known carcinogen, to form the monomer styrene, which is reasonably anticipated to be a human carcinogen. Apart from low cost, low strength foam, PS can be made as a clear, glassy, hard polymer used for things like cutlery and cd cases; also higher impact versions exist for harder applications. Properties: versatility, clarity, easily formed.
Typical Use: EPS: Styrofoam food containers, egg cartons, disposable cups and bowls, take-out food containers, deli food plates, packaging, packing peanuts, bike helmets. Harder clear/opaque PS: disposable cutlery & razors, compact disc & dvd cases. High impact PS: hangers, smoke detector housing, licence plate frames, medecine bottles, test tubes, petri dishes, model assembly kits.
Toxicity: PS food containers can leach styrene, which is reasonably anticipated to be a human carcinogen (PS1, PS2) and is considered a brain and nervous system toxicant (PS1, PS3, PS4). Animal studies have shown adverse effects on genes, lungs, liver, and the immune system (PS2). Note that styrene is also present in second-hand cigarette smoke, off-gassing building materials, and car exhaust. The leaching of styrene from PS containers into food is increased when the food or liquid is hot and oily (PS3, PS4) .
Recycling Rate: Very low, because difficult to recycle (PS5). Recycled material made into packaging and thermal insulation.
Alternatives: Avoid PS take-out containers - bring your own reusable dishes/containers for take-out. Buy in glass and reuse those bottles/jars - mason jars are incredibly versatile. Use reusable dishes (e.g., ceramic, stainless steel) and cutlery (e.g., stainless steel, bamboo) for picnics and events.
Our Suggestion: AVOID. Can leach styrene, which is a brain and nervous system toxin and likely carcinogenic.
 Other (O) - all other plastics
Other (O) - all other plastics
This category does not identify one particular plastic resin. It is a general catch-all for all plastics other than those identified by numbers 1-7, and can include plastics that may be layered or a mixture of various plastics. It includes the new bioplastics.
Polycarbonate (PC) is an extremely common plastic in this category and is often associated with this category (sometimes a product will have the number 7 on it with the letters "PC" underneath), so we describe it below -- But keep in mind that polycarbonate is not the only plastic in this category and if a product has a number 7 on it without the letters PC under it, the product could be made of polycarbonate or it could be any other plastic (and there are thousands!). The only way to know for sure is to ask the manufacturer or have the plastic tested.
Polycarbonate (PC)
Description: Polycarbonate use as a consumer plastic has decreased drastically in recent years due to the health-related problems associated with bisphenol A (BPA), the primary molecule in PC polymers, as well as increasing national bans on its use for certain products such a baby bottles and infant formula packaging. It is a tough family of engineering plastics originally developed to be an alternative to die-cast metal. It's strength and transparency made it a popular choice for consumer products needing to be shatter-proof, and also for epoxy resins. PC is also known by various trade names including Lexan, Makrolon and Makroclear. Properties: Easily molded, temperature resistance, stiffness, strength, optical clarity.
Typical Use: Baby bottles, sippy cups, water bottles, three and five gallon large water storage containers, metal food can liners, juice and ketchup containers, oven-baking bags, carbonless paper receipts. Also used in custom packaging, eye glass lenses, epoxy resins, dental sealants, compact discs, DVDs, Blu-ray discs, lab equipment, gears, snowboards, car parts, housing for cell phones, computers and power tools.
Toxicity: The problem with PC is bisphenol A (BPA), the synthetic backbone which readily breaks down and leaches from PC. For example, BPA leaching is a significant concern with PC epoxy-lined cans used for foods, especially oil-based and/or acidic foods, which will increase leaching. There is lots more information in our BPA section, but in a nutshell... BPA is often described as a hormone or endocrine disuptor, because it mimics human hormones, in particular the estrogen hormones, which are involved in normal cellular function, reproduction, development and behaviour. Peer-reviewed scientific studies have linked BPA to numerous health problems including chromosome damage in female ovaries, decreased sperm production in males, early onset of puberty, various behavioural changes, altered immune function, sex reversal in frogs, impaired brain and neurological functions, cardiovascular system damage, adult-onset (Type II) diabetes, obesity, resistance to chemotherapy, increased risk of breast cancer, prostate cancer, infertility, and metabolic disorders -- research into the impacts of BPA on human health is extensive and ongoing (PC1, PC2, PC3, PC4).
Recycling Rate: Very low (PC). Not all municipalities include polycarbonate as readily acceptable for their recycling programs. Recycled PC may be used to make plastic lumber.
Alternatives: Buy in glass and reuse those bottles/jars - mason jars are incredibly versatile. Use a glass or stainless steel reusable water bottle. Use a stainless steel water dispenser for large quantities of water or other liquids. If you must use the large blue PC bottles, transfer the water to another container as soon as you bring it home.
Our Suggestion for PC: AVOID. Leaches bisphenol A (BPA), which is a known endocrine disruptor with numerous adverse health effects, including increased risk of cancers.
You can find more detailed explanations of these and other plastics in our book:
A few other key references for the above text:
- Anthony L. Andrady, ed. Plastics and the Environment. Hoboken, NJ: John Wiley & Sons, 2003.
- Susan Freinkel. Plastic: A Toxic Love Story. New York: Houghton Mifflin Harcourt, 2011.
- Rick Smith & Bruce Lourie. Slow Death by Rubber Duck: How the Toxic Chemistry of Everyday Life Affects Our Health. Toronto: Alfred A. Knopf, 2009.
- E.S. Stevens. Green Plastics: An Introduction to the New Science of Biodegradeable Plastics. Princeton & Oxford: Princeton University Press, 2002.
- Beth Terry. Plastic Free: How I Kicked the Plastic Habit and How You Can Too. New York: Skyhorse Publishing, 2012.
- R. C. Thompson, C. J. Moore, F. S. vom Saal and S. H. Swan, eds. "Theme Issue: Plastics, The Environment and Human Health." Philosophical Transactions of the Royal Society B. Vol. 364, No. 1526, 27 July 2009.
- Michael Tolinski. Plastics and Sustainability: Towards a Peaceful Coexistence between Bio-based and Fossil Fuel-based Plastics. Salem, MA: Scrivener Publishing, 2012.
IMPORTANT NOTES: While we strive to provide as accurate and balanced information as possible on our website, Life Without Plastic cannot guarantee its accuracy or completness because there is always more research to do, and more up-to-date research studies emerging -- and this is especially the case regarding research on the health and environmental effects of plastics. As indicated in our Terms & Conditions, none of the information presented on this website is intended to be professional advice or to constitute a professional service to the individual reader. All matters regarding health require medical supervision, and the information presented on this website is not intended as a substitute for consulting with your physician.
Throughout our website, some technical terminology is used. In the interest of making the articles accessible and not too long, dry, or complex, technical terms may be hyper-linked to more detailed explanations and relevant reference material provided in Wikipedia. Please keep in mind that Wikipedia articles are written collaboratively by volunteers from all over the world and thus may contain inaccuracies. Life Without Plastic makes no guarantee of the validity of the information presented in Wikipedia articles to which we provide links. We suggest you read the Wikipedia General Disclaimer before relying on any information presented in a Wikipedia article.
© 2019 Mama Mundo Inc. All Rights Reserved. No part of this text may be reproduced without the prior written permission of Mama Mundo Inc.